- MindBio demonstrates safe, effective take home use of MB22001 in Depressed patients over an 8-week, world first take-home trial
- Rapid, robust, and clinically significant reduction in depression symptoms
- Primary efficacy endpoint achieved with an impressive mean 14.1 point drop in Montgomery-Asberg Depression Rating Scale ("MADRS") score
- After just 8 weeks, patients experienced a 60% reduction in depressive symptoms and 53% were remitted from Depression
- MB22001 was safe and well tolerated with no treatment-related severe or serious adverse events
VANCOUVER, BC / ACCESSWIRE / February 26, 2024 / MindBio Therapeutics Corp. (CSE:MBIO)(Frankfurt:WF6), (the "Company" or "MindBio"), a leading biopharmaceutical company in psychiatric medicine development, is delighted to announce positive top line data from its world-first take-home microdosing depression clinical trial using MB22001. The completion of this landmark and highly successful Phase 2a clinical trial is a major inflection point for the Company as it moves to late-stage pharma drug development.
Justin Hanka, Chief Executive Officer of MindBio said, "We are delighted to share that MB22001 showed rapid and statistically significant improvements with 60% reduction in depressive symptoms and 53% of patients experiencing complete remission from depression. The treatment resulted in an impressive mean 14.1 point drop in Montgomery-Asberg Depression Rating Scale ("MADRS"). These Phase 2 trial results are transformative for the Company as it takes its next steps into late-stage pharma".
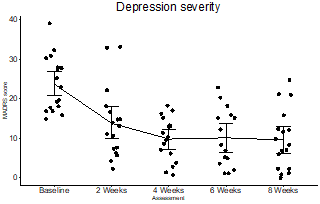
MindBio has achieved a significant milestone as the only organisation in the world that is running multiple clinical trials with Government and Regulatory approvals for take-home use and handling of a psychedelic medicine by trial patients out in the community, specifically a proprietary self-titratable form of Lysergic Acid Diethylamide (LSD) in microdoses designed for take home use (MB22001).
MindBio has collected tens of thousands of data points, over 3 years from clinical trials including psychometric data, speech analytics, sleep, biometric, activity data, EEG, ECG, PK/PD including DNA sampling. MindBio is building a proprietary treatment model that is scalable, safe and effective and can be tailored to patients as a first line treatment for Depression. Our proprietary drug candidate MB22001 is self-titratable, allowing patients to dose up or down depending on the individual's tolerance. The Phase 2a clinical trial demonstrated excellent safety, adherence and tolerance profile in doses tested. This was consistent with the Phase 1 trial results.
We invite you to join us in support of creating a brighter future for mental health.
Watch a brief explainer of the results here: https://youtu.be/DIB1YifPMwg
Receive our latest updates here: https://www.mindbiotherapeutics.com/get-updates
Follow MindBio on LinkedIn: https://www.linkedin.com/company/mindbio-therapeutics/?viewAsMember=true
Follow CEO Justin Hanka on LinkedIn: https://www.linkedin.com/in/justinhanka/
For further information, please contact:
Justin Hanka, Chief Executive Officer
61 433140886
justin@mindbiotherapeutics.com
Media Inquiries
Kristina Spionjak
pr@hlthcommunications.com
About MindBio Therapeutics
MindBio is a leading biotech/biopharma company focused on creating novel and emerging treatments for mental health conditions and is conducting world first take-home Microdosing (MB22001) human clinical trials. MB22001 is MindBio's lead candidate drug, a proprietary titratable form of Lysergic Acid Diethylamide (LSD) designed for take-home microdosing. MindBio is a leader in microdosing of psychedelic medicines and is advancing its drug and technology protocols through clinical trials. MindBio has developed a multi-disciplinary platform for developing treatments and is involved in psychedelic medicine development and digital therapeutics, has completed Phase 1 clinical trials in 80 healthy participants, has a Phase 2a clinical trial just completed microdosing in patients with Major Depressive Disorder and a Phase 2B clinical trial currently underway microdosing in late stage cancer patients experiencing existential distress. MindBio invests in research that forms the basis for developing novel and clinically proven treatments including digital technologies and interventions to treat debilitating health conditions such as depression, anxiety and other related mental health conditions.
Cautionary Note Concerning Forward-Looking Statements:
The press release contains "forward-looking statements" within the meaning of applicable securities laws. Forward-looking statements can be identified by words such as: "anticipate," "intend," "plan," "budget," "believe," "project," "estimate," "expect," "scheduled," "forecast," "strategy," "future," "likely," "may," "to be," "could," "would," "should," "will" and similar references to future periods or the negative or comparable terminology, as well as terms usually used in the future and conditional. Forward-looking statements are based on assumptions as of the date they are provided. However, there can be no assurance that such assumptions will reflect the actual outcome of such items or factors.
Additionally, there are known and unknown risk factors that could cause the Company's actual results and financial conditions to differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important risk factors that could cause actual results and financial conditions to differ materially from those indicated in the forward-looking statements, include among others: general economic, market and business conditions in Canada and Australia; market volatility; unforeseen delays in timelines for any of the transactions or events described in this press release. All forward-looking information is qualified in its entirety by this cautionary statement.
The Company disclaims any obligation to revise or update any such forward-looking statement or to publicly announce the result of any revisions to any of the forward-looking information contained herein to reflect future results, events or developments, except as required by law.
Neither the Canadian Securities Exchange nor its Regulation Service Provider (as that term is defined in the policies of the Canadian Securities Exchange) accepts responsibility for the adequacy or accuracy of this release.
SOURCE: MindBio Therapeutics
View the original press release on accesswire.com














