Reports Preliminary 2021 First Quarter Revenue and Business Update
Lucira Health, Inc. (Nasdaq: LHDX), a medical technology company focused on the development and commercialization of transformative and innovative infectious disease test kits, today announced the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for over the counter (OTC) sale of the LUCIRA CHECK IT™ test kit that delivers PCR quality molecular accuracy in 30 minutes or less at home. It is authorized and available for individuals with or without symptoms and can be ordered from lucirahealth.com for $55.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210412005361/en/
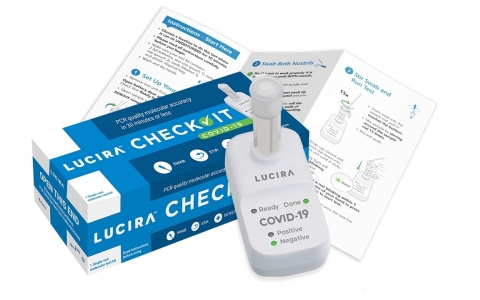
The LUCIRA CHECK IT™ test kit provides PCR quality, COVID-19 results in 30 minutes or less in the comfort of home. (Photo: Business Wire)
This primarily U.S. designed and manufactured product was the first FDA authorized, prescription, molecular diagnostic test for COVID-19 that could be self-administered by patients at home or used in a physician’s office. OTC clearance dramatically expands the availability of this highly accurate test.
Each single-use test kit contains everything needed to conduct one COVID-19 test. It can detect a positive result in as few as 11 minutes or confirm a negative result within 30 minutes. It was designed and tested extensively for individuals to use independently and does not require a physician’s prescription or telehealth / supervised assistance.
“We worked with more than 1,000 people before starting our FDA clinical and usability studies. Our goal was to produce an easy-to-use test that provides PCR quality accuracy in a portable, intuitive, anytime, anywhere format,” said Lucira CEO Erik Engelson. “People are looking for ways to feel more certain in these uncertain times, and our Lucira CHECK IT test provides that.”
To support people who would like to quickly receive confirmation of their test results for work and other needs, Lucira partnered with Converge Technology Solutions to develop a secure, text-based way for people with smartphones to receive a free LUCI Pass™ without downloading an app. The LUCI Pass was developed to support Lucira’s over-the-counter test kit and is unique to Lucira. Users simply text a short code to access LUCI, and then go through a simple sequence of steps including scanning their test result to receive a LUCI Pass and verified test to their phone. Results are also transmitted to the required public health authorities.
Sensitive, accurate, easy to use
In clinical trials, Lucira’s easy-to-use ‘swab, stir and detect’ CHECK IT test kit demonstrated that 100% of users successfully performed the test in about two minutes. Labs currently take two to fourteen days to generate similarly accurate test results.
Molecular tests are more sensitive than antigen tests because they amplify critical parts of the viral target. The targeted, molecular amplification that Lucira CHECK IT and PCR tests employ makes them demonstrably more sensitive and reliable than “rapid” antigen tests, which can miss active COVID-19 infections.
In a Community Trial setting, Lucira CHECK IT results were compared with the Hologic Panther Fusion, considered one of the highest sensitivity molecular tests due to its low Limit of Detection (LOD). Lucira’s accuracy was 98%, detecting 385 out of 394 positive and negative samples correctly when compared to the Hologic Panther Fusion, and excluding ten samples with very low levels of virus (those with very high PCR cycle thresholds of 37.5 or greater) that possibly no longer represented active infection. Comparative positive results agreed 97% of the time among this sample, and negative results agreed 98% of the time.
Preliminary 2021 First Quarter Revenue and Business Update
- Lucira expects net revenue for the three months ended March 31, 2021 of approximately $4.0 million to $4.5 million.
- Lucira added on-line ordering of its prescription product on Lucira’s website in addition to other channels.
- First quarter 2021 results will be reported on May 13, 2021.
“The first quarter of 2021 was very fluid for Lucira, as the rapid rollout of multiple COVID-19 vaccines, coupled with a temporary inventory build of COVID-19 testing kits and an overall slowdown in testing in the United States, hampered our growth in the point of care,” said Lucira CEO Erik Engelson. “Despite the dynamics that occurred in the first quarter, we believe our OTC LUCIRA CHECK IT Test Kit is well-positioned to take advantage of the shift to at home decentralized COVID-19 self-testing.”
LUCIRA CHECK IT Test Kit
The Lucira CHECK IT Test Kit fits in the palm of a hand, extracts genetic material from the virus and amplifies it similar to PCR lab tests. Each Lucira test kit contains everything needed to run one COVID-19 test. Users get the test device, two AA batteries, sample vial, swab and simple instructions. The batteries are inserted in the device and the sample vial is placed in the test unit. The user then opens the test swab packet and rotates the swab in each nostril five times. The swab is then stirred in the sample vial, which is then gently pressed into the test unit to start the test. The “ready” light will blink until a “positive” or “negative” green light is illuminated within 30 minutes. For guidance on care and public health reporting, people can use Lucira’s text based, secure LUCI portal to receive a result verification back on their phone while at the same time transmitting their result to the relevant public health authorities.
Lucira still has its identical, prescription product available for sale to healthcare providers at lucirahealth.com
About Lucira Health, Inc.
Lucira is a medical technology company focused on the development and commercialization of transformative and innovative infectious disease test kits. Lucira’s testing platform produces lab quality molecular testing in a single-use, consumer-friendly, palm size test kit powered by two AA batteries. Lucira designed its test kits to provide accurate, reliable and on-the-spot molecular test results anywhere and at any time. The LUCIRA CHECK IT (OTC) and LUCIRA COVID-19 All-In-One Test Kit (RX) are designed to provide a clinically relevant COVID-19 result within 30 minutes from sample collection.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include statements regarding, among other things, Lucira’s financial results (preliminary and unaudited) for the three months ended March 31, 2021. The data is not a comprehensive statement of Lucira’s results for this period, and Lucira’s actual results may differ materially from these preliminary estimated data. Lucira’s actual results remain subject to the completion of management’s and Lucira’s audit committee’s reviews and its other financial closing processes as well as the completion and preparation of its financial data for the three months ended March 31, 2021. During the course of the preparation of such financial statements and related notes, additional adjustments to the preliminary estimated financial information presented below may be identified, and Lucira’s final results for this period may vary from these preliminary estimates. This preliminary estimated data should not be considered a substitute for the financial statements to be prepared in accordance with accounting principles generally accepted in the United States and filed with the Securities and Exchange Commission (SEC). Accordingly, you should not place undue reliance on these preliminary data. Further, as forward-looking statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “expects,” “plans,” “believe,” “will”, “anticipates,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon Lucira’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks and uncertainties associated with Lucira’s business in general and the other risks described in Lucira’s filings with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made and are based on management’s assumptions and estimates as of such date. Lucira undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210412005361/en/
Contacts
Investor Contact
Greg Chodaczek
347-620-7010
investorrelations@lucirahealth.com
Media Contact
Kevin Knight
206-451-4823
media@lucirahealth.com













