– Continued positive trend in North Star Ambulatory Assessment (NSAA) scores relative to BMD natural history trajectories –
– Highly significant decreases in levels of serum creatine kinase (CK) and fast skeletal muscle troponin I (TNNI2), enzyme biomarkers strongly associated with muscle damage observed in BMD –
– EDG-5506 continues to be well-tolerated at higher doses –
– Edgewise leadership to discuss findings on Friday, October 14 at the Annual Congress of the World Muscle Society –
Edgewise Therapeutics, Inc. (NASDAQ: EWTX), a clinical-stage biopharmaceutical company focused on developing orally bioavailable, small molecule therapies for the treatment of rare muscle disorders, announced today positive 6-month interim results from the ongoing ARCH study, an open label, single-center study assessing the safety, tolerability, impact on muscle damage biomarkers, and pharmacokinetics (PK) of EDG-5506 in adults with BMD. EDG-5506 is an investigational orally administered small molecule myosin modulator designed to protect injury-susceptible fast skeletal muscle fibers in dystrophinopathies such as Duchenne muscular dystrophy (DMD) and BMD.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221013005743/en/
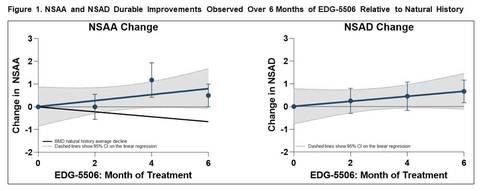
Figure 1. NSAA and NSAD Durable Improvements Observed Over 6 months of EDG-5506 Relative to Natural History (Graphic: Business Wire)
The twelve adults with BMD enrolled in the ARCH study initially received a 10 mg dose of EDG-5506 during the first 2 months, followed by a 15 mg dose during the subsequent 4 months of the study. The Company is reporting data at the end of 6 months of treatment with EDG-5506. Plasma PK levels reached target exposures and EDG-5506 continues to be well-tolerated in all participants with no discontinuations or dose reductions. The most common adverse events observed to date were dizziness, drowsiness, and headache. All participants have now been dose escalated to daily 20 mg oral doses of EDG-5506.
Consistent with prior observations, treatment with EDG-5506 led to a significant decrease in key biomarkers of muscle damage when assessed by laboratory assays. Importantly, CK and fast skeletal muscle troponin I were reduced by an average of 39% and 75%, respectively, after the participants most recent visit.
After 6 months of EDG-5506 dosing, NSAA scores continued to trend in a positive direction. Of note, a similar trend was observed with the North Star Assessment for Limb Girdle Type Muscular Dystrophies (NSAD) scores, an expanded functional outcome scale developed for adult populations which includes higher difficulty tasks. Eight of the twelve participants showed either a functional improvement or exhibited no decline on NSAA relative to their baselines. As seen in Figure 1, NSAA scores show a consistent positive trend that diverges from trajectories observed in the natural history study reported by Bello et al. (2016)1 in which the yearly decline was 1.22 NSAA points.
The Company believes the 6-month ARCH study data with EDG-5506 provide further support that reducing contraction-induced damage in dystrophic muscle has the potential to preserve and improve muscle function while preventing disease progression in dystrophinopathies. EDG-5506’s tolerability and efficacy observations to date have allowed the Company to explore accelerated ways to advance EDG-5506 into potential registrational trials.
“As a company, we are dedicated to the muscular dystrophy community and are thankful for the trial participants as we are critically evaluating this important data to help guide our clinical development plan in BMD and DMD,” said Joanne Donovan, M.D., Ph.D., Chief Medical Officer of Edgewise. “We continue to be encouraged by EDG-5506’s safety profile and the positive trends observed with these interim data.”
“BMD is a serious neuromuscular disorder and individuals living with this disease have no approved treatment options,” added Kevin Koch, Ph.D., President and Chief Executive Officer of Edgewise. “These early data are very encouraging and highlight EDG-5506’s potential to alter the course of the disease.”
Annual Congress of the World Muscle Society: Edgewise Presentation
On Friday, October 14, 2022, at 7 am ADT, Edgewise will sponsor a symposium, “Targeting Fast Muscle Myosin: a Novel Approach to Protecting Muscle for the Dystrophinopathies.” As part of the symposium, Dr. Donovan will present 6-month interim results from the ongoing ARCH study. Only registered conference attendees can register for the symposium. The symposium will be streamed to the virtual element of the event and available for replay via the conference platform. The Edgewise symposium presentations will be available on the Edgewise website when they are presented.
About the ARCH Open Label Study
The ARCH open label study is evaluating EDG-5506 in 12 adult males with BMD. All those who participated in the Phase 1b first-in-human study of EDG-5506 enrolled in the ARCH study after at least a 3-month washout following their participation in the Phase 1b trial. The study is evaluating escalating doses of EDG-5506 administered daily over 12 months. Safety, PK, changes in biomarkers of muscle damage such as CK and fast skeletal muscle troponin I, measures of function with NSAA and NSAD, time function tests and patient-reported outcomes are being evaluated. Go to clinicaltrials.gov to learn more about this study (NCT05160415).
About Becker Muscular Dystrophy
BMD is a serious, progressively debilitating, and potentially fatal inherited X-linked neuromuscular disorder. BMD results from mutation of the dystrophin gene yielding unstable and/or dysfunctional dystrophin expression in muscles. Individuals with BMD, typically males, have ongoing muscle fiber (myofiber) degeneration that eventually leads to fibrosis, progressive loss of skeletal muscle function, and that can lead to severe disability and early death. BMD typically presents with juvenile onset of muscle wasting and progressive symmetrical, proximal muscle weakness, calf hypertrophy, activity-induced muscle cramping and elevated creatine kinase activity. While the course of BMD is variable, it is unidirectional in terms of the inevitable progressive limb weakness resulting in severe disability. BMD is also associated with early mortality from cardiac disease. The incidence of BMD is approximately 1 in every 18,450 live male births. It is estimated that there are between 4,000 to 5,000 individuals with BMD in the U.S., with similar numbers of individuals living with BMD in the EU and UK. Despite the seriousness of the disease, for many with BMD, the disease remains one of considerable unmet medical need as there are no approved therapies in the U.S.
About EDG-5506
EDG-5506 is an orally administered small molecule designed to address muscle damage induced by mechanical stress in dystrophinopathies including DMD and BMD. EDG-5506 presents a novel mechanism of action designed to selectively limit the exaggerated muscle damage caused by the absence or loss of functional dystrophin. By impacting the progressive muscle damage that leads to functional impairment, EDG-5506 has the potential to benefit a broad range of patients suffering from debilitating rare neuromuscular disorders. It is anticipated to be used as a single agent therapy, but it may also provide a synergistic or additive effect in combination with available therapies and therapies currently in development. In August 2021, the U.S. Food and Drug Administration (FDA) granted Fast Track designation to EDG-5506 for the treatment of individuals with BMD.
The Company has completed a Phase 1 clinical trial of EDG-5506 designed to evaluate safety, tolerability, PK and pharmacodynamics of EDG-5506 in adult healthy volunteers (Phase 1a) and in adults with BMD (Phase 1b) (NCT04585464). In ARCH, a follow-on open-label, single-center trial (NCT05160415) assessing long-term safety and PK, decreases in biomarkers of muscle damage and improvements in NSAA have been observed. CANYON, a Phase 2 trial (NCT05291091) is assessing safety, PK, biomarkers and functional measures in participants with BMD. Recently, the FDA authorized LYNX, a Phase 2 trial (NCT05540860) of EDG-5506 for the treatment of DMD that will assess safety, PK and biomarkers of muscle damage.
About Edgewise Therapeutics
Edgewise Therapeutics is a clinical-stage biopharmaceutical company focused on the discovery, development, and commercialization of innovative treatments for severe, rare neuromuscular and cardiac disorders for which there is significant unmet medical need. Guided by its holistic drug discovery approach to targeting the muscle as an organ, Edgewise has combined its foundational expertise in muscle biology and small molecule engineering to build its proprietary, muscle-focused drug discovery platform. Edgewise’s platform utilizes custom-built high throughput and translatable systems that measure integrated muscle function in whole organ extracts to identify small molecule precision medicines regulating key proteins in muscle tissue. To learn more, go to: www.edgewisetx.com or follow us on LinkedIn, Twitter and Facebook.
References
[1] Bello L, et al., Functional Changes in Becker Muscular Dystrophy: Implications for Clinical Trials in Dystrophinopathies, Scientific Reports, 2016.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Statements in this press release that are not purely historical are forward-looking statements. Such forward-looking statements include, among other things, statements regarding the potential of, and expectations regarding, Edgewise’s drug discovery platform, product candidates and programs, including EDG-5506; statements regarding Edgewise’s expectations relating to its clinical trials and clinical development of EDG-5506; and statements by Edgewise’s president and chief executive officer and chief medical officer. Words such as “believes,” “anticipates,” “plans,” “expects,” “intends,” “will,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements. The forward-looking statements contained herein are based upon Edgewise’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those projected in any forward-looking statements due to numerous risks and uncertainties, including but not limited to: risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics and operating as an early clinical-stage company including the potential for Edgewise’s product candidates to cause serious adverse events; Edgewise’s ability to develop, initiate or complete preclinical studies and clinical trials for, obtain approvals for and commercialize any of its product candidates for muscular dystrophy patients or other patient populations; the timing, progress and results of clinical trials for EDG-5506; Edgewise’s ability to raise any additional funding it will need to continue to pursue its business and product development plans; negative impacts of the COVID-19 pandemic on Edgewise’s operations, including preclinical and clinical trials; the timing, scope and likelihood of regulatory filings and approvals; the potential for any clinical trial results to differ from preclinical, interim, preliminary, topline or expected results; Edgewise’s ability to develop a proprietary drug discovery platform to build a pipeline of product candidates; Edgewise’s ability to enroll and maintain patients in its ongoing and future clinical trials; Edgewise’s manufacturing, commercialization and marketing capabilities and strategy; the size of the market opportunity for Edgewise’s product candidates; the loss of key scientific or management personnel; competition in the industry in which Edgewise operates; Edgewise’s reliance on third parties; Edgewise’s ability to obtain and maintain intellectual property protection for its product candidates; general economic and market conditions; and other risks. Information regarding the foregoing and additional risks may be found in the section entitled “Risk Factors” in documents that Edgewise files from time to time with the Securities and Exchange Commission (the “SEC”). These forward-looking statements are made as of the date of this press release, and Edgewise assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20221013005743/en/
Contacts
Investors & Media
Michael Carruthers
Chief Financial Officer
ir@edgewisetx.com














