Kardium Inc. announces the successful first-in-human study of its next generation Globe® Pulsed Field System to treat atrial fibrillation (AF) using pulsed field ablation (PFA) therapy. Working with Dr. Vivek Reddy1 from Mount Sinai Hospital (New York, USA), and Prof. Petr Neužil and Dr. Jan Petrů from Na Homolce Hospital (Prague, Czech Republic), the Kardium® team successfully treated 38 patients, isolating 100% of the patients’ pulmonary veins using the Globe Pulsed Field System.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221108005508/en/
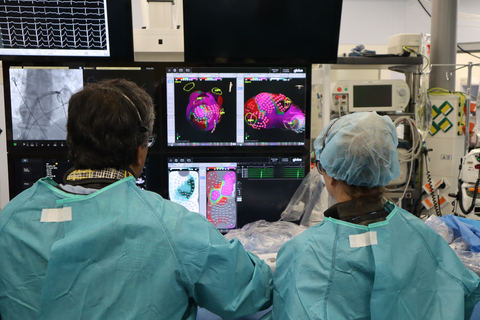
Dr. Vivek Reddy uses the Globe Pulsed Field System from Kardium to treat a patient with persistent Atrial Fibrillation (Photo: Business Wire)
The Globe Pulsed Field (PF) System features the revolutionary Globe Catheter with 122 gold electrodes, each of which can map the patient’s cardiac anatomy and electrical activity and deliver PFA energy to the heart to treat atrial fibrillation. The Globe Catheter uses contact sensing to determine which electrodes are in contact with cardiac tissue to help ensure therapy is delivered effectively to the heart.
In this study, patients with paroxysmal AF received pulmonary vein isolation (PVI) treatment in as little as 16 minutes. Patients with persistent AF received PVI treatment, and then the Globe PF System was used to create a high-definition map of the entire atrium using real-time 3-D mapping capabilities. Using these maps, all of the patients with persistent AF received additional ablation of the posterior wall and several patients also received a mitral isthmus line, all using the same Globe Catheter.
"PVI with the Globe PF System was very easy. I could quickly position the catheter in each pulmonary vein, ensure that the electrodes were in good contact, and then deliver pulsed field energy to isolate each vein safely and rapidly from one position,” said Dr. Reddy. “I also really liked the system's flexibility in more complex cases. I could map the atrium and then deliver additional pulsed field lesions to isolate the posterior wall quickly and then also create a mitral isthmus line.”
“The Globe PF System is designed to be a safe, effective and rapid treatment for AF,” said Kevin Chaplin, CEO of Kardium. “We are extremely excited by these procedures, which demonstrate the flexibility and ease of use of the Globe PF System. Previous clinical cases with the Globe PF System have achieved 100% durable PVI three months after the procedure.”
Kardium now plans to conduct a global pivotal clinical study of the Globe PF System in the US, Canada, Germany, and the Czech Republic for the purpose of obtaining regulatory approval for commercial sales.
About Kardium
Kardium Inc. (kardium.com) is a rapidly growing medical solutions company that has developed an advanced atrial fibrillation (AF) treatment system: the Globe Pulsed Field System.
The Globe PF System is an all-in-one solution for treating atrial fibrillation, which affects more than 59 million people worldwide. The Globe Catheter has 122 electrodes, each capable of high-definition mapping and delivering pulsed field ablation. The patented design of the Globe Catheter allows for rapid, single-shot pulmonary vein isolation (PVI), high-definition mapping, and atrial ablation.
1 - V.R. is a consultant to Kardium.
View source version on businesswire.com: https://www.businesswire.com/news/home/20221108005508/en/
Contacts
Kevin Chaplin, CEO, Kardium Inc.+1-604-248-8891 | kevin.chaplin@kardium.com













