Voom™ Medical Devices, Inc. (“Voom”), a rapidly growing orthopedic medical device company with a primary focus on pioneering innovative solutions for minimally invasive bunion surgery (MIBS), is thrilled to announce the introduction of its one-of-a-kind patented Revcon™ Anchor minimally invasive screw, along with the full commercial launch of the Bunionplasty® procedure.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20231017544531/en/
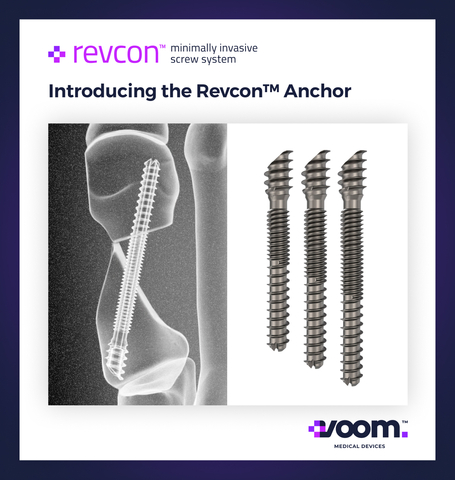
Voom™ Medical Devices launches the distinctive patented Revcon™ Anchor v2.0 screw, the only neutral non-compressive dual-zone pitched single screw solution for minimally invasive bunion surgery, exclusively used with the Bunionplasty® procedure. (Graphic: Business Wire)
The Revcon™ Minimally Invasive Screw System heralds a groundbreaking advancement, offering the exclusive No-Fusion Bunion Solution™ with patented screws tailored for minimally invasive bunion treatment. Designed by surgeons, for surgeons, both the Revcon™ Neutra v1.0 and our next advancement – the Revcon™ Anchor v2.0 – feature a neutral, non-compressive screw pitch, specially engineered to stabilize bone segments, fostering bony healing and regeneration, an essential aspect of MIBS.
“Our Revcon™ Anchor screw is an unparalleled product differentiator, representing a revolutionary solution for bunions,” says Dr. Neal Blitz, DPM, Founder and CEO of Voom. “The Revcon™ Anchor screw stands as the only dual-zone pitched screw explicitly designed for capturing different bone densities in bunion repair, using Voom’s proprietary Transveron™ bone cutting and realignment techniques.
The Bunionplasty® procedure utilizes Voom’s proprietary methods, now enhanced by the Revcon™ Anchor v2.0, in addition to the classic Revcon™ Neutra v1.0 screws. “Only surgeons trained on the Bunionplasty® procedure will have access to the Revcon™ Screw System technology,” Blitz adds. “We are attracting the field’s most talented surgeons, who seek to get their patients back on their feet fast, with tiny incisions like the ones used in laparoscopic surgery.”
Both screws are part of the Revcon™ Minimally Invasive Screw System, and have received FDA clearance for various foot indications and osteotomies, particularly for hallux valgus (bunion) treatment.
Since its inception in 2018, Voom has been dedicated to the advancement of fusion-free bunion solutions. “Non-fusion is more than a surgical choice,” says Blitz. “It has been our core value at Voom since day one, evident in our product design, surgeon training, professional thought leadership, and patient education.”
Voom is already reshaping perspectives among surgeons who perform bunion repair and other foot and ankle procedures. Dr. Lawrence DiDomenico, DPM, Voom’s Director of Medical Education, contends that the finest surgeons are those who continue to evolve and invest in their careers. “As a pioneer in the Lapidus surgery movement, I no longer believe we have to resort to fusion as the answer, in a vast majority of bunion treatments,” he says. “The combination of the Revcon™ Screw System and the Bunionplasty® procedure is a seismic shift. It offers an incredible opportunity for surgeons and their patients, and I anticipate that many will entirely abandon Lapidus as the standard, along with companies that provide Lapidus-centric solutions.”
Voom expects that as patient awareness of MIBS continues to rise, the demand for surgeons who are proficient in its No-Fusion Bunion Solution™ will rise sharply as well. “With the launch of Voom’s distinctive Revcon™ Anchor v2.0, it's evident that we are the leaders in minimally invasive bunion surgery innovation. We’re passionately committed to providing access to the best care for patients who seek out our Bunionplasty® procedure,” says Blitz. “This is who we are. This is what it means to Voom.”
For more information about Voom Medical Devices and its revolutionary innovations, please visit www.voomdevices.com or contact info@voomdevices.com.
About Voom Medical Devices, Inc.
Voom Medical Devices, Inc. is a rapidly growing orthopedic medical device company specializing in innovative solutions for minimally invasive bunion surgery (MIBS). Founded in 2018, Voom is committed to advancing fusion-free bunion solutions, promoting surgical innovation, and ensuring the highest quality of patient care. Visit www.voomdevices.com to learn more.
View source version on businesswire.com: https://www.businesswire.com/news/home/20231017544531/en/
Groundbreaking advancement in minimally invasive bunion surgery with Voom Medical Devices distinctive no-fusion bunion solution™ with its launch of the patented Revcon™ Anchor screw, with the only single screw solution dual-zone pitched screw.














