- The United States Food and Drug Administration (FDA) has awarded orphan drug designation (ODD) to NM5072 for the treatment of anemia in PNH patients.
- The ODD provides NM5072 with a range of benefits, including financial incentives, regulatory support, extended market exclusivity, and extended patent life, ensuring that this groundbreaking treatment can reach those who need it.
- NM5072 is an anti-Properdin antibody that selectively blocks the alternative pathway (AP) while maintaining the integrity of the classical pathway required for fending off infections.
- The drug has completed phase I trial in 48 healthy volunteers with a clean safety profile and was well-tolerated across all doses tested up to 20 mg/kg. Total AP inhibition was achieved, with its duration exhibiting a dose-dependent response.
- NM5072 is being positioned to enter Phase II trial for the treatment of naïve PNH patients as the first clinical indication.
- The drug is expected to be useful in other complement-mediated and complement-associated disorders, further expanding the patient population for multiple clinical trials.
CLEVELAND, April 15, 2024 (GLOBE NEWSWIRE) -- NovelMed today announced that the Food and Drug Administration (FDA) has awarded Orphan Drug Designation (ODD) to NM5072, an Alternative Pathway (AP) blocker anti-Properdin antibody, for the treatment of patients with Paroxysmal Nocturnal Hemoglobinuria (PNH).
NM5072 is being developed for PNH patients in the United States and globally. The FDA grants ODD status to new medication intended for the treatment, diagnosis or prevention of rare diseases or disorders that affect fewer than 200,000 people in the United States.
PNH is a rare, chronic, inflammatory, and hemolytic disease that occurs when PNH Red Blood Cells (RBCs) breakdown within and outside the blood vessel, causing anemia, which can be fatal. In addition to PNH RBCs, other cells, including Neutrophils, Monocytes, and Platelets, also drive cellular destruction and inflammation, leading to long-term effects on the patient’s health. If the disease remains untreated or partially treated, patients will continue to have anemia and inflammation, potentially leading to chronic pain, fatigue, and other common symptoms of PNH.
Dr. Rekha Bansal, Chief Executive Officer said, “PNH is a rare disease which involves a range of blood cells that contribute to debilitating symptoms for patients, including anemia, fatigue, and severe pain with shorter life span if remains untreated. The current standard of care requires further improvements. We are hopeful that NM5072, with its unique mechanism of action that targets the top of the complement cascade, could become a promising treatment to improve outcomes in these patients.” NM5072 is under regulatory review for multiple indications in the USA and globally.
“We are thrilled by the FDA’s decision, which highlights the demand for groundbreaking therapies for individuals grappling with PNH,” comments Robert Bard, VP Regulatory Affairs. This Orphan Drug Designation approval represents a remarkable stride in therapeutic innovation, showcasing NM5072’s distinct capability to selectively inhibit the alternative pathway (AP) while safeguarding the classical pathway essential for combating infections in PNH patients. NM5072 is another drug in our pipeline that has received Orphan Drug Designation in the United States.
Orphan Drug Designation (ODD)
The ODD from the FDA is a special designation granted to facilitate the development and assessment of new medicines. The FDA’s Office of Orphan Products Development has granted ODD for NM5072 which is in development for the treatment and prevention of rare diseases or conditions affecting fewer than 200,000 people in the US. This designation offers various advantages which include the potential for market exclusivity lasting seven years upon FDA approval, eligibility for tax credits related to qualified clinical trials, waiver of application fees, reduced annual product fees, clinical protocol assistance, and the potential qualification for expedited development programs.
NM5072 (Anti-Properdin Monoclonal Antibody)
NM5072 is being developed by NovelMed as a potential first-in-class monoclonal antibody that selectively blocks parts of the immune system responsible for the disease, thus inhibiting alternative pathway cascades. Blocking Properdin function is expected to block the lysis of PNH RBCs, which are implicated in the pathophysiology of anemia in PNH.
NM5072 is currently an investigational drug that selectively binds to Properdin, a protein of the AP. This monoclonal antibody serves as an efficacious blocker of the AP without affecting the part of the immune system required for fighting infections.
NM5072 has successfully completed a phase I trial in healthy volunteers with an expected safety profile. This potent drug is in various regulatory phases of development. With its much longer half-life, the Anti-Properdin molecule is a pioneering biologic. “While the drug is currently in the form of an IV formulation, subcutaneous formulations are being planned for future trials,” said Dr. Bansal. Regulatory preparations and review of Phase II trials for multiple indications, including PNH, C3 Glomerulopathy (C3G) and Atypical Hemolytic Uremic Syndrome (aHUS) are underway.
Complement-Mediated Disorders
The dysregulation of the Alternative Pathway (AP) is a pivotal factor influencing the trajectory of numerous acute and chronic rare diseases, spanning disciplines such as hematology, ophthalmology, nephrology, dermatology, and neurology. Among these, Paroxysmal Nocturnal Hemoglobinuria (PNH) emerges as a prominent indication for complement blockers heading towards FDA approval.
In the context of PNH, the current armamentarium of treatment options remains limited and imperfect, often compromising pathogenic clearance or inducing hyperlipidemia. PNH manifests with a spectrum of clinical features, including low PNH-RBC clone size, hemolysis, hemoglobinuria, reduced hemoglobin levels, elevated lactate dehydrogenase (LDH), and a plethora of other symptoms that can precipitate chronic complications, potentially leading to premature mortality if left untreated. Chronic symptoms and multisystem organ damage are recurrent in PNH patients.
While FDA-approved anti-C3, C5, and Factor B drugs provide potential for symptom management in PNH treatment, they should be approached with caution due to their significant side effects and broader limitations on labels. The imperative to develop additional treatment modalities for PNH, characterized by both efficacy and safety, remains unequivocally recognized, holding the promise of enhancing disease outcomes and improving the quality of life for affected individuals.
About NovelMed
Dedicated to pioneering innovative biologics for a range of complement-mediated diseases, NovelMed stands at the forefront of conceptualizing and validating an anti-Properdin antibody for chronic complement-mediated and complement-associated disorders. Progressing its leading product candidate through clinical trials, the company primarily focuses on addressing hemolytic and renal disorders initially.
“Driven by a commitment to ingenuity and compassion, NovelMed is committed to delivering groundbreaking diagnostics and therapies that redefine the treatment landscape for patients with rare diseases,” said Dr. Rekha Bansal, Chief Executive Officer. Positioned as a frontrunner in the development of therapies targeting the Alternative Pathway (AP), NovelMed is devoted to addressing debilitating diseases across hematology, ophthalmology, Nephrology, dermatology, and neurology.
Our R&D team is currently engaged in the development of a new diagnostic marker aimed at identifying complement-mediated diseases. This synergistic strategy is geared towards broadening the spectrum of diseases that can be effectively and confidently treated using NM5072.
Seeking Partnership to Advance the Development of Drug candidates for Rare Diseases -
NovelMed is actively seeking licensing, investment, partnership, and acquisition opportunities to propel NM5072 through further development and approval across multiple rare disease indications in the US and globally. For more information about NM5072, please visit www.NovelMed.com/news.
Contact:
Ya Gao
BD@novelmed.com
(216) 440-2696
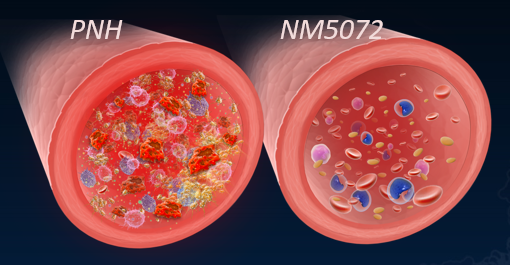
The expected efficacy of the NM5072 treatment
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/f0a478f7-1f00-4ca5-8951-6f50c93d7930
















