 Heart disease equipment maker Edwards Life Sciences (NYSE: EW) stock has fallen (-46%) on the year after a disastrous reaction to its Q3 2022 earnings report. The maker of heart valve systems and repair products for both surgical and transcatheter therapies reported a small miss and lowered its full-year 2022 guidance and got punished hard for it. While it may appear to be an overreaction at first, a glance at its valuation compared to peers indicates just how expensive shares were heading into the earnings report and is still expensive after shaving (-20%) of its share price afterward. A closer look at the historical price action of the shares illustrates the punishment that shareholders have endured as its stock has gone through three back-to-back stock plunges after each bounce attempt failed as shares lost nearly half its value from the top. Cost inflation and interest rate headwinds made a material impact on its recent earnings. While based in Irvine, California, Edwards sells its products in almost 100 countries worldwide. The strong U.S. dollar definitely impacted its earnings as its aortic valve sales rose 1% but actually 5% in constant currency in its recent quarter. Hospital staffing shortages in the U.S. and COVID restrictions in Japan contributed to the shortfall as well as the lowered guidance for the rest of 2022. These constraints appear to be more transitory than permanent. Underneath the gloomy sentiment is a silver lining as Edwards is a key player in the growing epidemic and business of combating heart disease among the aging population.
Heart disease equipment maker Edwards Life Sciences (NYSE: EW) stock has fallen (-46%) on the year after a disastrous reaction to its Q3 2022 earnings report. The maker of heart valve systems and repair products for both surgical and transcatheter therapies reported a small miss and lowered its full-year 2022 guidance and got punished hard for it. While it may appear to be an overreaction at first, a glance at its valuation compared to peers indicates just how expensive shares were heading into the earnings report and is still expensive after shaving (-20%) of its share price afterward. A closer look at the historical price action of the shares illustrates the punishment that shareholders have endured as its stock has gone through three back-to-back stock plunges after each bounce attempt failed as shares lost nearly half its value from the top. Cost inflation and interest rate headwinds made a material impact on its recent earnings. While based in Irvine, California, Edwards sells its products in almost 100 countries worldwide. The strong U.S. dollar definitely impacted its earnings as its aortic valve sales rose 1% but actually 5% in constant currency in its recent quarter. Hospital staffing shortages in the U.S. and COVID restrictions in Japan contributed to the shortfall as well as the lowered guidance for the rest of 2022. These constraints appear to be more transitory than permanent. Underneath the gloomy sentiment is a silver lining as Edwards is a key player in the growing epidemic and business of combating heart disease among the aging population.Addressing Heart Disease
Heart disease is the leading cause of death in the U.S. responsible for 697,000 deaths in 2020, one out of every five deaths. Aortic stenosis (AS) is the narrowing of the aortic valve caused by calcium buildup over time. Risk factors for the development of AS include diabetes, chronic kidney disease, high blood pressure and abnormal lipids. This can cause a blockage of blood flow to the body thereby forcing the heart to work harder. It’s the most common valvular heart disease and is present in nearly 5% of the population at age 65 and increases with age. Transcatheter aortic valve replacement (TAVR) or valve implementation (TAVI) is used to treat AS. The procedure replaces a damaged aortic valve with a manufactured valve made of pig or cattle (bovine) tissue. Edwards currently sells the SAPIEN 3, SAPIEN 3 Ultra and SAPIEN 3 Ultra RESILIA Transcatheter Heart Valve System. Its RESILIA tissue contains advanced calcium blocking technology which has the potential to improve valve longevity and reduce intervention. Heart valves usually last up to 10-years. RESILIA tissue has been shown to lower calcium content by 72%. Edwards also utilizes RESILIA in many of its surgical valves for treatment of mitral valve disease, when the valves don’t close tightly in the left heart chambers causing blood to leak backwards.
Valuation, Not Earnings Killed the Stock
On Oct. 22, 2022, Edwards released its fiscal third-quarter 2022 results for the quarter ending September 2022. The Company reported an earnings-per-share (EPS) profit of $0.61 excluding non-recurring items versus consensus analyst estimates for a profit of $0.62, a (-1%) miss. Revenues rose 0.7% year-over-year (YoY) to $1.32 billion missing analyst estimates for $1.33 billion. Discontinued HARPOON program resulted in a charge of $0.07 per share. Transcatheter Aortic Valve Replacement (TAVR) sales rose 1% YoY to $862 million or 6% on a constant currency basis. Sales were mostly impacted by hospital staffing shortages in the U.S. and COVID headwinds in Japan. Edwards Life Sciences CEO Michael Mussallem commented, "The third quarter strengthened our conviction in our company's patient-focused innovation strategy. Despite a challenging macro environment, our team made significant progress advancing our breakthrough technologies. Our rigorous evidence generation has resulted in new global product approvals of innovative therapies, which will enable us to reach many more patients." Edwards also announced it executed an accelerated $750 million stock buyback to bring total share buybacks to over $1.7 billion in 2022. There is still another $900 million remaining in the share repurchase authorization approved by its Board of Directors.
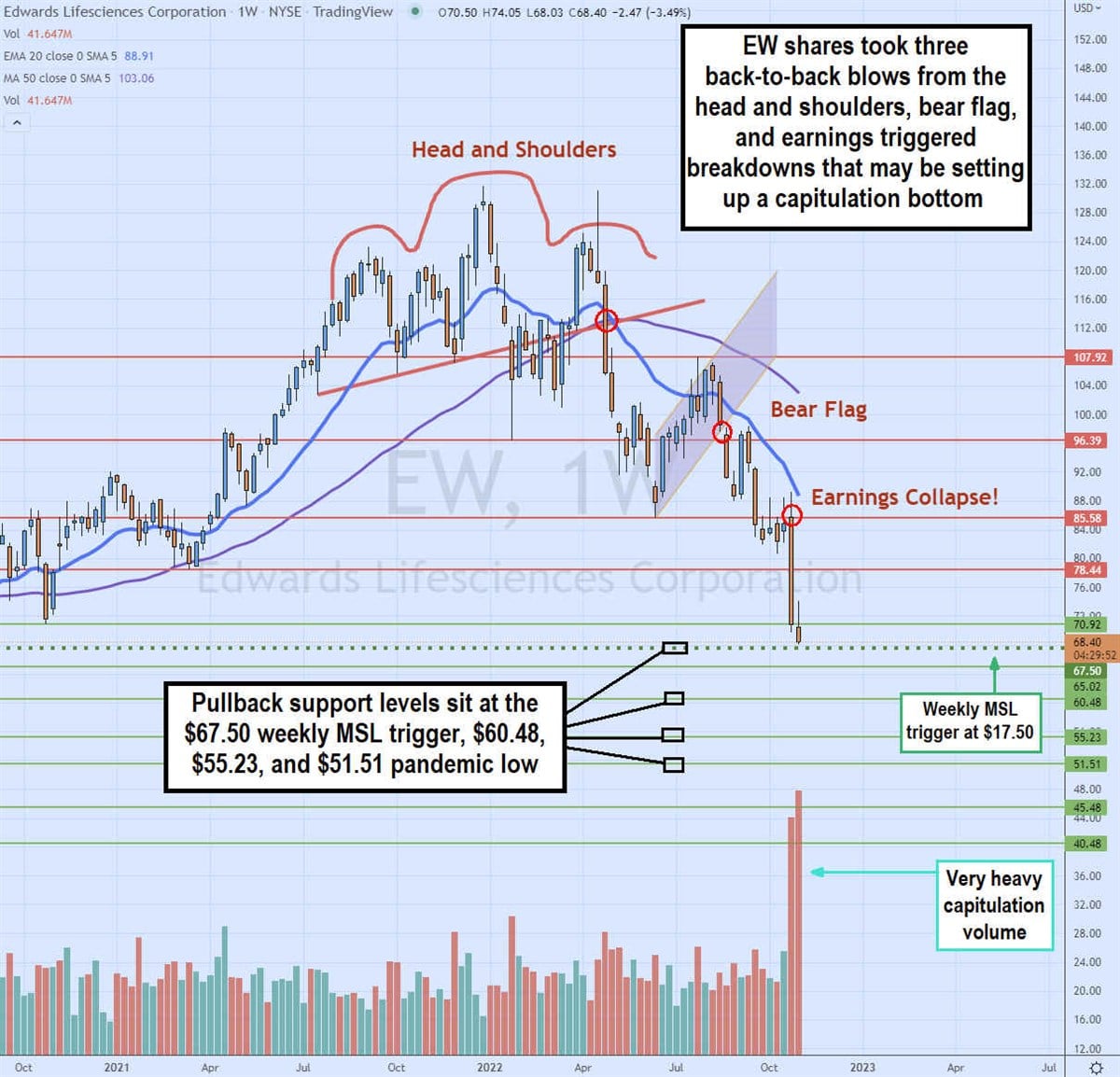
Three Strikes and Bulls are Out?
The weekly chart on EW is painful to watch as it got hit hard with two consecutive bearish patterns back to back. First the head and shoulders pattern triggered when the neckline collapse at $112.84 to fall down to $85.58. EW then staged a rally off those lows up to $107.92, which gave the bulls hope for a further rally. Unfortunately, it was a trap as the bear flag that triggered a rug pull on the breakdown of $98.34 causing shares to sink to $80.69 before staging a small rally up to $89.17 in anticipation of earnings. Unfortunately (again) the Q3 2022 earnings game up short causing the stock to plunged towards the pandemic market structure low trigger level at $70.92 on the heaviest weekly volume since the pandemic lows, which could be a sign of capitulation. Three strikes comprised of a weekly head and shoulders breakdown, a bear flag breakdown, and an earnings disaster collapse could mean the bulls are throwing in the towel and out. Pullbacks can be watch for at the $68.40 weekly market structure low trigger, 67.50 support level, $60.48, $55.23 and 51.51 support levels.
Pipeline Approvals to Drive Growth
Edwards received U.S. FDA and European CE Mark approval for PASCAL Precision for treatment of degenerative mitral regurgitation in September and August, respectively. The system is designed for transcatheter-based-edge-to-edge leaflet repair for sufferers of mitral and tricuspid regurgitation. The Company has been approved to begin selling the SAPIEN 3 Ultra RESILIA valve in the U.S. Enrollment accelerated in its 2 TAVR pivotal trials for its next-gen SAPIEN X4. CEO Mussallem commented, “These transformative developments reinforce our confidence in the continued growth of transcatheter-based structural heart inventive. We will continue to aggressively pursue breakthrough technologies with the potential to help even a broader group of patients and in turn drive significant future value.”
Shares Have More Room to Fall
Edwards lowered its guidance for fiscal full-year 2022 EPS of $2.40 to $2.50 from $2.50 to $2.65 versus $2.52 consensus analyst estimates. Full-year 2022 revenues are expected to come in between $5.35 billion to $5.55 billion versus $5.47 billion consensus analyst estimates. Edwards Lifesciences shares trade at 28X forward earnings, nearly twice that of rival Medtronic (NYSE: MDT) trading at 15X forward earnings. It still trades at a higher valuation than other medical device maker peers like Boston Scientific (NYSE: BSX) at 24X, Becton Dickinson at (NYSE: BDX) at 19.3X, and St. Jude Medical owned by Abbott Labs (NYSE: ABT) at 18.5X. The market is repricing its valuation leaving it more room to fall to get in line with its peer trading closer to a 18X to 24X forward earnings. It becomes a value play once shares fall under the 20X forward earnings mark.














