SiO2 Materials Science, a privately owned U.S.-based advanced materials science company, today released the findings from their new report: Glass-Related Recalls in Pharma: 2014 - July 2021
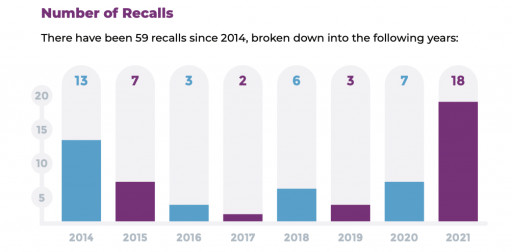
The report collected publicly available recall information from the FDA and European regulators. The report then identified 59 instances where drugs were recalled due to glass-related issues between January 2014 and July 2021. The causes identified include recalls for delamination, particulate matter, breakage, ethylene oxide residuals and adhesive issues.
"This year we've already seen 18 recalls due to glass-related problems," said Lawrence Ganti, President at SiO2 Materials Science. "Until the industry breaks free from being dependent on borosilicate glass packaging, a material that was invented 100 years ago and hasn't evolved since, we will continue to see more recalls, and more importantly, we'll continue to see patients' health and safety put at risk. This report confirms our belief that it's time for the pharma industry to rethink the use of glass."
To access a full report with an analysis of each of the 59 recalls, please visit: https://www.sio2ms.com/glass-related-recalls-in-pharma-2014-july-2021/
About SiO2 Materials Science:
SiO2 Materials Science is an advanced materials science corporation introducing breakthrough disruptive technology serving the biopharma, molecular diagnostic, and consumer healthcare industries. The company is located in Auburn, Alabama. The company has deep partnerships with leading professors at the foremost research universities such as the University of California - Santa Barbara, University of Chicago, and MIT. For more information, visit www.sio2ms.com.
Media contact:
Brett@Gofrontlines.com
Press Release Service by Newswire.com
Original Source: New Report Finds 59 Drugs Have Been Recalled Due to Glass-Related Issues Since 2014