Bioavailability of buprenorphine up to 20-fold higher in preclinical models using prodrug generated with the Glyph technology, driven by transport of prodrug through the gut-draining lymphatics
The research published in Frontiers in Pharmacology further demonstrates the ability of the Glyph technology to improve bioavailability and lymphatic targeting of a range of clinically validated drugs, including buprenorphine
PureTech Health plc (Nasdaq: PRTC, LSE: PRTC) ("PureTech" or the "Company"), a clinical-stage biotherapeutics company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases, today announced the publication of preclinical proof-of-concept demonstrating that PureTech’s Glyph platform can enhance the oral bioavailability of buprenorphine (BUP), a clinically-validated opioid replacement therapy, further expanding the range of clinically-validated drug classes shown to be amenable to the Glyph technology. The paper, published in Frontiers in Pharmacology1, applies the Glyph technology to BUP, a potent analgesic that is widely used for severe pain management and opioid replacement therapy but is not currently available in an ingestible oral dosage form due to poor oral bioavailability. In preclinical models, the researchers observed increases in bioavailability of up to 20-fold and statistically significant increases in lymphatic transport.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220411005986/en/
PureTech announced the publication of preclinical proof-of-concept showing up to 20-fold oral bioavailability enhancement by the Glyph platform of buprenorphine, a clinically-validated opioid replacement therapy. The paper was published by PureTech collaborator, Christopher Porter, Ph.D., Director of the Monash Institute of Pharmaceutical Sciences at Monash University in Melbourne, in the journal Frontiers in Pharmacology. (Photo: Business Wire)
“The therapeutic potential of buprenorphine is potentially limited by a lack of systemic exposure after the administration of a capsule formulation that can be swallowed. The ability to develop an oral buprenorphine product with high bioavailability could potentially address a range of important unmet clinical needs and offer more convenience for patients,” said Christopher Porter, Ph.D., Director of the Monash Institute of Pharmaceutical Sciences at Monash University in Melbourne, lead author of the study and PureTech collaborator. “Results from this study further amplify the breadth of the Glyph delivery technology and its ability to use new chemistry and molecules for versatile applications.”
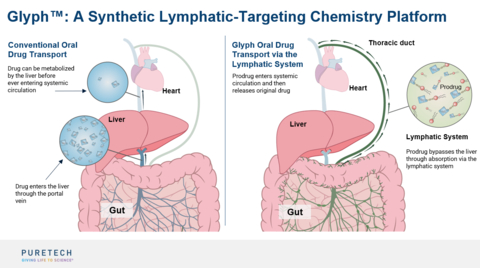
The Glyph technology generates novel orally dosed prodrugs by reversibly linking small molecule drugs to dietary fat molecules. This linkage is designed to channel the drugs directly into the systemic circulation via the lymphatic system, thereby bypassing first-pass liver metabolism which typically degrades many drugs and reduces their systemic exposure. The Glyph technology is being developed to be applicable to a range of clinically validated drugs with poor bioavailability, including neuromodulators such as allopregnanolone (with the clinical-stage therapeutic candidate, LYT-300) or immune modulators that could directly target the mesenteric lymph nodes.
“The research serves as another proof-of-concept for our Glyph platform and how this innovative drug delivery technology can be applied to a range of diseases,” said Joseph Bolen, Ph.D., Chief Scientific Officer of PureTech. “This latest research reinforces our commitment to leveraging validated biology to accelerate the development of the Glyph portfolio to improve the oral bioavailability and/or lymphatic targeting of proven drugs.”
PureTech’s LYT-300 is the first therapeutic candidate generated by the Glyph technology platform to enter the clinic. LYT-300 is an oral formulation of the clinically validated neurosteroid allopregnanolone, in development for the potential treatment of a range of neurological and neuropsychological conditions. An injectable formulation of allopregnanolone is approved by the United States Food and Drug Administration (FDA) for the treatment of postpartum depression as a 60-hour infusion, a method of administration that has inherent limitations. Synthetic oral analogs of allopregnanolone have had variable clinical success, and their comparable activity with natural allopregnanolone remains to be established.
About the Glyph™ Technology Platform
The Glyph technology is PureTech's synthetic lymphatic-targeting chemistry platform which is designed to employ the lymphatic system's natural lipid absorption and transport process to enable the oral administration of therapeutics. The Glyph technology reversibly links small molecule drugs to dietary fat molecules, creating a novel prodrug. The linked fat molecule re-routes the drug's normal path to the systemic circulation, bypassing the liver and instead moving from the gut into the lymphatic vessels that normally process dietary fats. PureTech believes this technology has the potential to (1) enable direct modulation of the immune system via drug targets present in mesenteric lymph nodes and (2) provide a broadly applicable means of enhancing the bioavailability of orally administered drugs that would otherwise be reduced by first-pass liver metabolism. PureTech is leveraging validated biology to accelerate the development of a Glyph portfolio, prioritizing highly characterized drugs to enhance with the Glyph technology based on the potential value unlocked in improving their oral bioavailability or lymphatic targeting. PureTech's lead Glyph therapeutic candidate, LYT-300 (oral allopregnanolone), is being evaluated in a Phase 1 study, with results expected in the second half of 2022. PureTech has exclusively licensed the Glyph technology platform, which is based on the pioneering research of Christopher Porter, Ph.D., and his research group at the Monash Institute of Pharmaceutical Sciences at Monash University. The Porter Research Group and collaborators have published research in Frontiers in Pharmacology, Nature Metabolism and the Journal of Controlled Release supporting the Glyph platform's ability to directly target the lymphatic system with a variety of therapies.
About PureTech Health
PureTech is a clinical-stage biotherapeutics company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases, including inflammatory, fibrotic and immunological conditions, intractable cancers, lymphatic and gastrointestinal diseases and neurological and neuropsychological disorders, among others. The Company has created a broad and deep pipeline through the expertise of its experienced research and development team and its extensive network of scientists, clinicians and industry leaders. This pipeline, which is being advanced both internally and through PureTech's Founded Entities, is comprised of 25 therapeutics and therapeutic candidates, including two that have received both U.S. FDA clearance and European marketing authorization, as of the date of PureTech's most recently filed Half Year Report and corresponding Form 6-K. All of the underlying programs and platforms that resulted in this pipeline of therapeutic candidates were initially identified or discovered and then advanced by the PureTech team through key validation points based on the Company's unique insights into the biology of the brain, immune and gut, or BIG, systems and the interface between those systems, referred to as the BIG Axis.
For more information, visit www.puretechhealth.com or connect with us on Twitter @puretechh.
Cautionary Note Regarding Forward-Looking Statements
This press release contains statements that are or may be forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation those statements that relate to expectations regarding the potential therapeutic benefits of our therapeutic candidates or platform technologies, our expectations regarding the Glyph platform and PureTech’s future prospects, development plans and strategies. The forward-looking statements are based on current expectations and are subject to known and unknown risks, uncertainties and other important factors that could cause actual results, performance and achievements to differ materially from current expectations, including, but not limited to, those risks, uncertainties and other important factors described under the caption "Risk Factors" in our Annual Report on Form 20-F for the year ended December 31, 2020 filed with the SEC and in our other regulatory filings. These forward-looking statements are based on assumptions regarding the present and future business strategies of the Company and the environment in which it will operate in the future. Each forward-looking statement speaks only as at the date of this press release. Except as required by law and regulatory requirements, we disclaim any obligation to update or revise these forward-looking statements, whether as a result of new information, future events or otherwise.
[1] Hu, L., Quach, T., Han, S., Lim, S.F., Senyschyn, D., Trevaskis, N.L., Simpson, J.S., Porter, C.J.H. Self immolative glyceride mimetic prodrugs promote lymphatic transport, avoid first pass metabolism and enhance bioavailability. Angew. Chem. Ind. Ed. (2016) 55, 13700-13705.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220411005986/en/
Contacts
PureTech
Public Relations
publicrelations@puretechhealth.com
Investor Relations
IR@puretechhealth.com
EU Media
Ben Atwell, Rob Winder
+44 (0) 20 3727 1000
ben.atwell@FTIconsulting.com
U.S. Media
Nichole Sarkis
+1 774 278 8273
nichole@tenbridgecommunications.com













