The 25-week weight loss study which achieved its previously reported primary endpoints also examined the effects of GS200 on insulin resistance
Gelesis Holdings Inc. (NYSE: GLS) (“Gelesis” or the “Company”) the maker of Plenity for weight management, today released a poster presentation at the International Congress of Endocrinology in Singapore. The LIGHT-UP study evaluated the safety and efficacy of GS200, an investigational oral hydrogel, which was designed to emulate the properties of ingested raw vegetables, with slightly different mechanical properties compared to Plenity (GS100).
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220825005222/en/
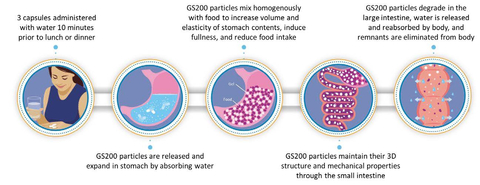
GS200 hydrogel in the gastrointestinal tract. (Graphic: Business Wire)
The LIGHT-UP study was conducted over 25 weeks in 254 participants with prediabetes or type 2 diabetes and a BMI of 27-40 kg/m2. It met its primary endpoints and GS200 reported a highly favorable categorial weight loss response and tolerability in a population that often struggles to lose weight and is at high risk for obesity-related complications. One out of three GS200-treated adults were “super responders,” losing at least 10% of their body weight and on average losing 13% (~30 pounds), or 7 inches off their waist circumference in only 25 weeks.
“In previous studies we observed better weight loss response for individuals with prediabetes. In this study we wanted to understand if we could replicate this intriguing finding also with people who have type 2 diabetes, since typically weight loss is even more challenging for them” said Frank L. Greenway, MD, Medical Director and Professor at the Pennington Biomedical Research Center, Louisiana State University and one of the study’s lead investigators. “These findings could help us to understand the reason why the hydrogel therapy works better for people with elevated insulin resistance, which is important given the heterogeneity of the mechanisms driving weight gain and obesity.”
The study also investigated meal-time insulin release in people with prediabetes using a two-hour oral glucose tolerance test. Participants with prediabetes treated with GS200 had significantly less total mealtime insulin release, compared to placebo, the mean difference was -22.0%, P=0.04. The peak level of mealtime insulin was also significantly lower for people that used GS200 when compared to placebo with a mean difference of 47.3μU/mL, P=0.03.
“These reductions in mealtime insulin release were statistically significant even after controlling for changes in weight loss between GS200 and placebo, indicating that the observed improvements are independent of weight loss, suggesting additional mechanisms involved in the metabolic effects of the oral hydrogel technology,” said Elaine Chiquette, Gelesis Chief Scientific Officer. “These are exciting results, and we continue to investigate how GS200 affects insulin response and weight loss in people with prediabetes and type 2 diabetes.”
About Gelesis
Gelesis Holdings Inc. (NYSE: GLS) (“Gelesis”) is a consumer-centered biotherapeutics company and the maker of Plenity®, which is inspired by nature and FDA cleared to aid in weight management. Our first-of-their-kind non-systemic superabsorbent hydrogels are made entirely from naturally derived building blocks. They are inspired by the composition and mechanical properties of raw vegetables, taken by capsule, and act locally in the digestive system, so people feel satisfied with smaller portions. Our portfolio includes Plenity® and potential therapies in development for patients with Type 2 Diabetes, Non-alcoholic Fatty Liver Disease (NAFLD)/Non-alcoholic Steatohepatitis (NASH), and Functional Constipation. For more information, visit gelesis.com, or connect with us on Twitter @GelesisInc. Plenity® is indicated to aid weight management in adults with excess weight or obesity, a Body Mass Index (BMI) of 25–40 kg/m², when used in conjunction with diet and exercise.
About Gelesis’ LIGHT-UP Clinical Study
The multicenter, double-blind, randomized, placebo-controlled study enrolled 254 subjects and was designed to assess the change in body weight in adults with overweight or obesity, who have prediabetes or diabetes, after 25 weeks of treatment with a new oral superabsorbent hydrogel (GS200) or placebo. The study met both of its primary endpoints: the proportion of participants who achieved at least 5% body weight loss and the change in body weight after six months of therapy.
A highly binary effect was observed with the GS200 treatment group, with a clear separation between responders and non-responders as early as after 6 weeks of treatment. Among the adults who completed the study protocol requirements (PP population), 64% of GS200-treated adults were Responders vs. 41% in the placebo group (p=0.001). In the analysis which also included data from the participants who didn’t fully complete the study (ITT-MI), 55% of GS200-treated adults were Responders vs. 34% in the placebo group (p=0.0004). The average body weight loss of the Responders was 11% (approximately 23 pounds) and their waist circumference was reduced by 5.5 inches on average. Importantly, Gelesis treated individuals had 2.8 higher odds compared with placebo to become Responders (adjusted odds ratio = 2.83, P=0.0004), achieving the first primary endpoint of the study.
With respect to average total weight loss, the complete GS200 treatment group (including both Responders and Non-Responders) demonstrated superiority over placebo after 25 weeks of treatment (body weight loss of 7.1% vs. 4.6%, P=0.0029 in the PP population or 6.9% vs. 4.3%, P=0.0011 in the ITT population), thereby achieving the second primary endpoint.
GS200 demonstrated a highly favorable safety and tolerability profile as the overall incidence of adverse events (AEs) in adults treated with GSP200 was similar to the incidence of AEs in the placebo group.
Important Safety Information about Plenity
- Patients who are pregnant or are allergic to cellulose, citric acid, sodium stearyl fumarate, gelatin, or titanium dioxide should not take Plenity.
-
To avoid impact on the absorption of medications:
- For all medications that should be taken with food, take them after starting a meal.
- For all medications that should be taken without food (on an empty stomach), continue taking on an empty stomach or as recommended by your physician.
- The overall incidence of side effects with Plenity was no different than placebo. The most common side effects were diarrhea, distended abdomen, infrequent bowel movements, and flatulence.
- Contact a doctor right away if problems occur. If you have a severe allergic reaction, severe stomach pain, or severe diarrhea, stop using Plenity until you can speak to your doctor.
Rx Only. For the safe and proper use of Plenity or more information, talk to a healthcare professional, read the Patient Instructions for Use, or call 1-844-PLENITY.
Forward-Looking Statements
Certain statements, estimates, targets and projections in this press release may constitute “forward-looking statements” within the meaning of the federal securities laws. The words “anticipate,” “believe,” continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “strive,” “would” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that statement is not forward looking. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and uncertainties. Forward-looking statements include, but are not limited to, statements regarding Gelesis’ or its management team’s expectations, hopes, beliefs, intentions or strategies regarding the future, including those relating to Gelesis’ expected operating and financial performance and market opportunities. In addition, any statements that refer to projections, forecasts, or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. Forward-looking statements speak only as of the date they are made. Readers are cautioned not to put undue reliance on forward-looking statements, and Gelesis assumes no obligation and does not intend to update or revise these forward-looking statements, whether as a result of new information, future events, or otherwise. Gelesis gives no assurance that any expectations set forth in this press release will be achieved. Various risks and uncertainties (some of which are beyond Gelesis’ control) or other factors could cause actual future results, performance or events to differ materially from those described herein. Some of the factors that may impact future results and performance may include, without limitation: (i) the ability of Gelesis to raise financing, if and when needed; (ii) the ability of Gelesis to continue as a going concern; (iii) Gelesis’ ability to achieve and maintain widespread market acceptance of Plenity; (iv) the impact of current and future applicable laws and regulations and Gelesis’ ability to comply with such laws and regulations; (v) Gelesis’ ability to produce adequate supply of Plenity, including Gelesis’ ability to continue to invest in manufacturing capacity and to build additional manufacturing sites; (vi) the development of the telehealth market and regulations related to remote healthcare; (vii) global economic, political and social conditions and uncertainties in the markets that Gelesis serves, including risks and uncertainties caused by the COVID-19 pandemic or other natural or man-made disasters; (viii) Gelesis' ability to enter into strategic collaborations, to acquire businesses or products or form strategic alliances and to realize the benefits of such collaborations, acquisitions and alliances; (ix) the level of demand, and willingness of potential members to pay out-of-pocket for, Plenity; (x) the ability of Gelesis to enforce its intellectual property rights and proprietary technology ; (xi) the risk that a third-party’s activities, including with respect to third parties that Gelesis has granted outlicenses to or granted limited exclusive or non-exclusive commercial rights, may overlap or interfere with the commercialization of Plenity; (xii) Gelesis’ ability to successfully develop and expand its operations and manufacturing and to effectively manage such growth; (xiii) Gelesis’ business partners' ability to successfully launch and commercialize Plenity in certain key markets; (xiv) risk relating to the loss of Gelesis’ suppliers or distributors, or their inability to provide adequate supply of materials or distribution; (xv) the risk that Gelesis’ business partners may experience significant disruptions in their operations; (xvi) Gelesis’ ability to retain its senior executive officers and to attract and keep senior management and key scientific and commercial personnel; (xvii) Gelesis’ ability to identify and discover additional product candidates and to obtain and maintain regulatory approval for such candidates; (xviii) risks related to potential product liability exposure for Plenity or other future product candidates; (xix) risks related to adverse publicity in the weight management industry, changes in the perception of Gelesis’ brands, and the impact of negative information or inaccurate information about Gelesis on social media; (xx) Gelesis’ ability to enhance its brand recognition, increase distribution of Plenity and generate product sales and reduce operating losses going forward; (xxi) the impact of risks associated with economic, financial, political, environmental and social matters and conditions on Gelesis’ supply chain, its manufacturing operations and other aspects of its business; (xxii) Gelesis’ ability to accurately forecast revenue and appropriately monitor its associated expenses in the future; (xxiii) Gelesis’ ability to compete against other weight management and wellness industry participants or other more effective or more favorably perceived weight management methods, including pharmaceuticals, devices and surgical procedures; (xxiv) foreign currency fluctuations and inflation; (xxv) the risk that Gelesis fails to maintain adequate operational and financial resources or to raise additional capital or generate sufficient cash flows; (xxvi) Gelesis’ ability to successfully protect against security breaches and other disruptions to its information technology structure; (xxvii) the ability of Gelesis to maintain its listing on the New York Stock Exchange; (xxviii) failure to realize the anticipated benefits of the business combination; and (xxix) other important factors discussed in the “Risk Factors” section of Gelesis’ most recent Annual Report on Form 10-K and in other filings that Gelesis makes with the Securities and Exchange Commission. These filings address other important risks and uncertainties that could cause actual results and events to differ materially from those contained in the forward-looking statements.
Source: Gelesis Holdings, Inc.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220825005222/en/
Contacts
Investors: ir@gelesis.com
Media: pr@gelesis.com













