– ALTO-101 demonstrated a favorable pharmacokinetic and tolerability profile; novel transdermal formulation delivered significantly greater drug exposure and fewer adverse events typically associated with PDE4 inhibitors –
– First-in-human achievement of target exposure with proprietary transdermal patch formulation developed in partnership with MEDRx –
– Proof-of-concept study in cognitive impairment associated with schizophrenia (CIAS) to be initiated in the first half of 2024 –
Alto Neuroscience, Inc. (“Alto”) (NYSE: ANRO) today announced positive results from its healthy volunteer Phase 1 study of ALTO-101, a novel PDE4 inhibitor in development for cognitive impairment associated with schizophrenia (CIAS). Results from the study demonstrated favorable tolerability and improved pharmacokinetics of ALTO-101 when administered via a transdermal delivery system (TDS) compared to oral administration. The TDS formulation is being developed in partnership with MEDRx. The drug exposure levels achieved with the transdermal formulation were significantly greater than systemic exposure with oral administration while also reducing typical class-wide adverse events. The Company plans to initiate a proof-of-concept study evaluating ALTO-101 in patients with CIAS in the first half of 2024, with topline data expected in the second half of 2025.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240423861340/en/
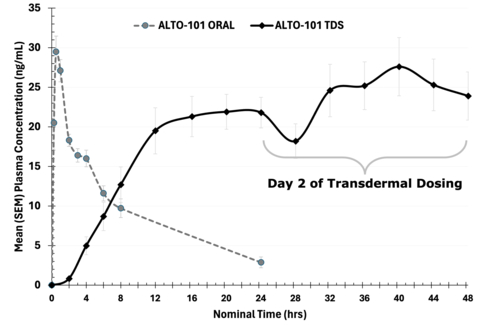
The pharmacokinetic curve illustrates stable drug exposure and achievement of the desired plasma concentration with the transdermal formulation as compared to orally administered ALTO-101. (Graphic: Alto Neuroscience, Inc.)
“These positive results provide a strong indication that ALTO-101 could become an important therapeutic option for a broad range of indications,” said Amit Etkin, M.D., Ph.D., founder and chief executive officer of Alto Neuroscience. “Oral PDE4 inhibitors have shown promise as pro-cognitive therapeutics but are associated with burdensome and dose-limiting side effects. Despite these dosing and tolerability challenges, PDE4 inhibitors are used widely in other non-CNS indications, often with sub-optimal dosing regimens. We have now demonstrated transdermal administration of ALTO-101 results in significantly greater, stable drug concentration with a once-daily formulation that is well-tolerated. We believe the reduction in typical class-wide adverse events observed with ALTO-101 provides a significant point of differentiation compared to other PDE4 inhibitors currently available, or in development. We look forward to advancing this product candidate for patients with CIAS who currently have few treatment options.”
Phase 1 Study Design and Results
The study was designed to evaluate the safety, tolerability, pharmacokinetics, and adhesion properties of a proprietary transdermal formulation of ALTO-101 compared to oral administration of ALTO-101 in healthy human volunteers. It included a 2-way crossover design consisting of two dosing periods. Fifteen adults between 40-64 years old were enrolled in the study. In period 1, participants received a single oral dose of 1 mg ALTO-101, following a 7-day washout period; in period 2, participants received continual transdermal dosing of 18 mg ALTO-101 for two days – patches were administered once-daily and worn for 24 hours. In both periods pharmacokinetics and tolerability were evaluated during and following treatment to measure absorption and elimination.
Topline Results from the ALTO-101 Phase 1 study include:
-
ALTO-101 delivered transdermally resulted in significantly higher, and consistent, drug concentrations compared to oral administration:
- Area under the curve (AUC) was 62% and 170% higher on day 1 and day 2 respectively for the transdermal formulation relative to oral administration (day 1 p=0.01; day 2 p<0.001).
- The maximum concentration (Cmax.) on day 2 of the transdermal dosing was similar to the Cmax of 1mg of orally administered ALTO-101 (27.9 ng/mL for TDS vs. 30.1 ng/mL oral).
- Plasma concentrations for the transdermal formulation remained stable throughout the 24 hours of dosing on day 2.
-
ALTO-101 delivered transdermally resulted in substantially lower class-related adverse events typically associated with PDE4 inhibitors.
- Dizziness - 40% oral vs. 7.1% TDS
- Nausea - 20% oral vs. 0% TDS
- All adverse events reported in the study were mild in severity, and there were no reported serious adverse events. No participants discontinued the study due to adverse events.
- The transdermal formulation demonstrated favorable adhesion properties and there were no application site reactions that led to patch removal or intolerance.
Overall, the results from this Phase 1 study indicate a favorable profile for ALTO-101 with higher levels of drug exposure, expected to be within the necessary therapeutic range, and lower rates of adverse events. The pharmacodynamic effects observed with orally administered ALTO-101, which were previously reported by the Company, inform the relevant therapeutic range believed to be required to achieve the desired pro-cognitive and clinical effects.
Under the terms of the development agreement between Alto and MEDRx, upon this achievement of the desired pharmacokinetic profile for ALTO-101, MEDRx is eligible to receive a milestone payment of $1.5 million from Alto that is payable in a combination of cash and common stock of Alto Neuroscience.
About ALTO-101
ALTO-101 is a novel small molecule PDE4 inhibitor being developed for cognitive impairment associated with schizophrenia, a disease state defined by negative and cognitive symptoms with no currently available targeted treatments. Through a proprietary transdermal delivery system, ALTO-101 is designed to provide steady state concentrations to improve drug safety, tolerability, and pharmacokinetics. The proprietary transdermal delivery system for ALTO-101 has been developed in partnership with MEDRx. In Phase 1 clinical trials, ALTO-101 demonstrated human brain penetration, robust CNS-relevant pharmacodynamic effects, and was well tolerated across therapeutically relevant dose ranges.
About Alto Neuroscience
Alto Neuroscience is a clinical-stage biopharmaceutical company with a mission to redefine psychiatry by leveraging neurobiology to develop personalized and highly effective treatment options. Alto’s Precision Psychiatry Platform™ measures brain biomarkers by analyzing EEG activity, neurocognitive assessments, wearable data, and other factors to better identify which patients are more likely to respond to Alto product candidates. Alto’s clinical-stage pipeline includes novel drug candidates in depression, PTSD, schizophrenia, and other mental health conditions. For more information, visit www.altoneuroscience.com or follow Alto on X.
Forward Looking Statements
This press release may contain forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements may be identified by words such as “aims,” “anticipates,” “believes,” “could,” “estimates,” “expects,” “forecasts,” “goal,” “intends,” “may,” “plans,” “possible,” “potential,” “seeks,” “will” and variations of these words or similar expressions that are intended to identify forward-looking statements, although not all forward-looking statements contain these words. Forward-looking statements in this press release include, but are not limited to, statements regarding Alto’s expectations for ALTO-101, the timing for the initiation of the ALTO-101 proof-of-concept trial, and the timing of the readout from the trial. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various factors, including the risk that Alto’s expectations concerning ALTO-101 which could cause Alto’s actual results to differ from those contained in the forward-looking statements, which are described in greater detail in the section titled “Risk Factors” in Alto’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023 filed with the Securities and Exchange Commission (“SEC”) as well as in other filings Alto may make with the SEC in the future. Any forward-looking statements contained in this press release speak only as of the date hereof, and Alto expressly disclaims any obligation to update any forward-looking statements contained herein, whether because of any new information, future events, changed circumstances or otherwise, except as required by law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240423861340/en/
Contacts
Investor Contact
Nick Smith
investors@altoneuroscience.com
Media Contact
Jordann Merkert
media@altoneuroscience.com













